Sharepoint Solutions
PRODUCTS
-
Scientific knowledge to strategize viable and efficient drug and device development pathways to achieve successful outcomes.
-
CAI’s Kneat: Data Ready provides you with best-in-class content and templates created by industry-leaders, giving you a headstart in implementing your own Kneat solution.
Kneat: Data Ready offers not only templates, but a validated out-of-the-box configuration that you can tailor to your business needs. Implement your Kneat instance in less than half the time.
-
Take control of your production by tracking every part, assembly, and batch with precision.
-
With over 130 in-life gene therapy studies completed, Altasciences is a leading expert in gene therapy research, with integrated preclinical and bioanalytical capabilities.
-
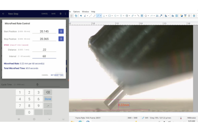
NOVA™ Software Upgrade Kit adds Enhanced Microfeed with programmable rates down to 5 µm/min, plus pop-up prompts and improved folder/program management.
WHITE PAPERS AND CASE STUDIES
-
What Does The New FDA DHT Guidance Mean?
In December 2023, the FDA released the final version of its digital health technologies guidance, outlining the facilitated use of sensor-based DHTs and wearables in clinical investigations.
-
The Role Of A Consulting Firm In The IND Process
Navigate the complex IND process with expert guidance and discover how early FDA engagement can accelerate your path to clinical trials.
-
Project Orbis Drug Registration 2025
Project Orbis helps streamline the review and approval of oncology medicinal products through international regulatory collaboration. Learn how this framework can accelerate your regulatory strategy.
-
eSource Purpose Is Not Just To Complete The EDC
Central eSource transforms data capture from a passive, back-end chore into an active, real-time quality and compliance engine—delivering benefits that extend beyond faster EDC feeds and reduced SDV.
-
Advancing In Silico Predictions In Early Drug Discovery
Examine a high-throughput approach that streamlines LogD measurements, offering faster and more accurate insight into compound lipophilicity to support better decision-making in early drug discovery.
-
AMERICAN Ductile Iron Pipe Used In Northwest Missouri's New Regional Water Authority
What began as a lofty idea will soon become a reality as a new regional water authority is established in Northwest Missouri. The Great Northwest Wholesale Water Commission will supply water to more than 11,000 people in rural areas of Cameron and Maysville, Missouri, with the capability to expand to other communities in the future.
-
Designing, Selecting, And Developing Bioconjugates For Clinical Success
Accelerate the development of complex bioconjugates to unlock new possibilities for targeted therapies and advance the next generation of precision medicine.
-
Quantitation Of Monoclonal Antibodies In Serum
Monoclonal antibodies (mAbs) are a rapidly growing group of targeted therapeutics. Gain insight into the importance of optimized bioanalytical assay platforms for mAb development.
-
Flexible Integrations To Reduce Risks And Increase Efficiencies
Learn how a trial team kept the blind of central lab samples and patient treatment across a Phase III and extension trial, whilst optimizing the supply chain.
-
Digitalizing Batch Records In Pharmaceutical Production
Pharma manufacturing embraces cutting-edge automation, yet outdated paper-based documentation hinders efficiency and delays batch releases. See how digital solutions can transform compliance.
-
Rescuing Data Integrity: A Swift Transition And Quality Transformation
Uncover how this collaboration supported timely progress toward key study milestones, enabling smoother execution and more dependable output across data management and statistical activities.
-
Aseptic Filling Solution Enhances Sterility Assurance for Pharma Manufacturer
Emergent BioSolutions upgraded its Winnipeg site with the Cytiva SA25 aseptic filling workcell to improve sterility assurance, product flexibility, and regulatory compliance for CGT manufacturing.
NEWS
-
Klarna Announces Partnership With OnePay To Exclusively Power Installment Loans At Walmart In The U.S3/17/2025
Klarna, the AI-powered payments and commerce network, today announced that it will be partnering with OnePay, a leading consumer finance app, to exclusively offer installment loans for purchases at Walmart in the United States.
-
Interior Cuts Red Tape To Speed Water Infrastructure In The West11/25/2025
The Department of the Interior today announced Secretary's Order 3446, which streamlines federally funded construction projects at Bureau of Reclamation facilities across the 17 Western states.
-
Canadian Drinking Water At Risk Long After Wildfires, UBC Study Warns3/4/2026
Canada’s drinking water can remain at risk long after wildfires burn out, according to a UBC-led global review that found water-quality impacts often emerge months or years later—not just immediately after a fire.
-
Securing Water Systems Against Cyber-Threats: Idaho Leads With Cyber-Informed Engineering3/6/2025
When it comes to cybersecurity research, the Idaho National Laboratory (INL) and Idaho have a relationship that dates back to the 1990s and was hallmarked in 2019 with the opening of the state-funded Cybercore Integration Center on INL’s Research and Education Campus in Idaho Falls.
-
Partnerships Drive Wheat Production And Livelihoods In Zimbabwe9/3/2025
Phaphamani Irrigation Scheme in Umguza District, Matabeleland North Province, Zimbabwe, yesterday showcased the transformative power of strong partnerships in boosting wheat production and improving rural livelihoods.