Capture
PRODUCTS
-
SpecVision™ is an in-line video inspection probe for Krell polishers, allowing surface viewing while parts stay loaded to reduce handling and contamination.
-
Mycenax develops and establishes high-quality cell lines for various host cell and product types. We are the experts for all cell line development activities, from vector construction to generating cell banks for GMP manufacturing following ICH and global regulatory requirements. We also support early-stage material generation for proof-of-concept studies or preclinical development programs.
-
Allogeneic CAR-T Cells Vs Autologous
Chimeric antigen receptor (CAR) T cell therapy has emerged as one of the major breakthroughs in cancer immunotherapy in the last decade. Clinical trials with autologous CAR-T cells have shown very promising results and led to the approval of numerous CAR-T cell therapies.
-
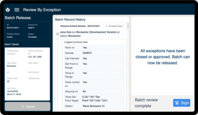
Enforce standardized workflows while collecting data in real time, giving you the visibility you need to prevent deviations, review exceptions, and release batches faster.
-
Advance the potential of your cell culture
Cedex Bio Analyzer - a reliable, high-quality instrument for small sample throughput
- Small size: Benchtop system suitable for any space
- Easy handling: Sophisticated and intuitive operating software
- Integrated processing unit: Built-in touchscreen reduces qualification effort
- Reliable answers: Robust and proven technology
- Efficiency: Low maintenance effort and frequency save time and money
WHITE PAPERS AND CASE STUDIES
-
Improving Methodologies For iPSC Manufacturing And Differentiation
Learn how next-generation workflows are being developed to streamline iPSC manufacturing, enhance differentiation strategies, and overcome the hurdles of scalability and consistency.
-
Assuring Multipotency Of Human Mesenchymal Stem Cells (hMSCs)
Stem cell research has revolutionized tissue repair and regenerative medicine. Discover how mesenchymal stem cells play a key role and offer insights into connective tissue repair, immune response, and inflammatory diseases.
-
Disinfection For Purified Water Loop For Kamada Pharmaceuticals, Israel
An Atlantium HOD™ (Hydro-Optic Disinfection) UV system was installed at the outlet of a PW tank to serve as the primary disinfection barrier, with water circulating through the system at 6m³/hr before reaching the points of use.
-
Enhancing Novel Developability Through Automated MS Analytics
Growing biotherapeutic complexity demands MS workflows that can unify diverse datasets. Learn how automated approaches enhance data quality, streamline analysis, and support faster development.
-
Robust, Scalable Strategy For Monodisperse Nanosuspensions Of Poorly Soluble Drugs
By tailoring the formulation strategy, selecting the appropriate excipients, and fine-tuning process conditions, see how our team was able to improve bioavailability and in vivo exposure.
-
Facilitate Handling Of Bulk Powders With Dry Granulation
Handling bulk powders in manufacturing presents serious challenges—from unpredictable caking to safety risks—that can disrupt workflows. Discover solutions to streamline operations and protect your team.
-
Flexible Data Management For Growing Portfolio
Explore strategic enhancements to a small biopharma's biometrics capabilities involving the deployment of 20 specialized professionals, which effectively bolstered the client’s organizational capacity.
-
Vial Adapter Transfer Device Compatibility With Cell Therapies
Needles and syringes are commonly used to retrieve cell therapies from vials, posing the risk of needle stick injury. Explore the suitability of needle-free polycarbonate transfer devices.
-
Designing And Evaluating A Water Treatment Media Pilot
Evaluating the use of activated carbon and other media for water treatment is a crucial step to ensure project goals are achieved.
-
How To Adopt CSA For Streamlined Computer System Validation
Discover how the FDA’s Computer Software Assurance guidance modernizes validation by reducing compliance burdens and fostering innovation through a streamlined approach to system validation.
-
Microbial Challenge In-Use Studies
Learn more about how a partner with expertise can help you navigate the complex landscape of microbial challenge in-use studies and ensure the highest standards of patient care.
-
Targeting Macular Degeneration With Stem Cell Therapy
A novel stem-cell therapy based upon iPSC-derived RPE cell transplantation was conceptualized as a promising new treatment for AMD and other retinal diseases.
NEWS
-
Fast Code Reader For Simple And Reliable Sample Identification2/18/2026
Meet the Micronic Rack Reader DR515, a compact, simple and high-performance 2D code scanning solution designed to enhance efficiency and accuracy in modern laboratory environments.
-
Oukitel Shapes The Future Of Rugged Technology With World-First Devices At IFA 20258/27/2025
Oukitel, a global leader in rugged technology, has consistently delivered top-tier devices designed to exceed users' highest expectations. At IFA 2025, it will unveil latest groundbreaking rugged devices, marking a milestone on the world's biggest stage for consumer electronics.
-
Parasoft Enhances C/C++ Software Test Automation With Full MISRA C:2025 Support, New AI-Driven Productivity, And Deeper Security Coverage6/17/2025
Parasoft, a global leader in AI-automated software testing solutions, raises the bar for safety-critical compliance with important enhancements to the company’s flagship static and dynamic testing solution for C and C++ software developers.
-
AsReader's New 'AsCameraX' Aims To Enhance Barcode Scanning Power Via Employee Smartphones7/17/2025
AsReader, a global leader in Barcode Scanning and RFID technology, has announced its new and improved AsCameraX, the company's newest software solution that can transform Android smartphones into powerful scanning tools.
-
SFT New Arrival Android 15 Rugged 5G Mobile Computer SF550P1/20/2026
SFT is happy to introduce their new launching of Android 15 Rugged Mobile Computer, an upgrading Android and processor device engineered to meet the demands of harsh industrial environments.