Capture
PRODUCTS
-
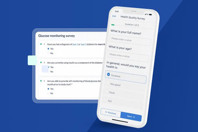
Capture data at the true source: the patient. Improve the patient experience with secure, accessible questionnaires both via email and through the Castor Connect mobile application.
-
Pharmaceutical Metal detectors identify small particles of stainless steel, ferrous, and non-ferrous metals that can enter the workflow from the manufacturing process, or raw materials.
-
Our injectable plants offer pre-filled water for injection (WFI) syringes in different sizes and volumes, produced according to European Pharmacopoeia (Ph. Eur.) and the United States Pharmacopeia (USP) requirements.
-
Immersion corrosion testing is a method used to determine the rate of corrosion of a test article, often a metal, in aqueous solution. Though this test can be used as an assessment tool for many applications, it is commonly used to evaluate the corrosivity of liquids.
-
Code and approve clinical trial data from any device and keep your safety and efficacy data clean, consistent, and ready for analysis.
WHITE PAPERS AND CASE STUDIES
-
Scalable Production Of High-Purity Nanobodies Using Yeast Expression System
Leverage scalable K. pastoris expression systems to produce high-quality nanobodies for cutting-edge therapeutic, diagnostic, and research applications.
-
Antibody-Drug Conjugates: 'Magic Bullets' Become Reality
Cancer therapy has evolved from potent chemotherapy to targeted biological therapy, including antibody-drug conjugates (ADCs). Learn more about ADCs’ makeup, mechanisms, and the development landscape.
-
Flow Chemistry Vs. Batch Processes
Explore how continuous flow chemistry can accelerate your chemical development with greater efficiency, safety, and sustainability compared to traditional batch processes.
-
Pasteurized Equivalent Water For AgriMark-Cabot Dairy In Vermont
Discover how AgriMark-Cabot Dairy transformed wastewater into a valuable resource using UV technology, achieving significant energy and water savings while maintaining stringent food safety standards.
-
Choosing The Right PCD Configuration For Reliable Sterility Assurance
Accurate PCD configuration ensures sterility assurance. Learn how to align validation strategies with ISO standards, optimize cycle efficiency, and select the right BI format for EO sterilization success.
-
The City Of Fayetteville's Flood Resiliency In The Face Of Climate Change: Mapping 15 Watersheds
The city of Fayetteville, North Carolina has always had flooding issues, but it’s been getting worse as weather patterns have been changing. They were hit four years in a row by storms Matthew (2016), Irma (2017), Florence (2018), and Dorian (2019).
-
How To Unlock The Secret To Repeatable, Scalable Low Turnover
Here, we explore the evolution of a 10-year partnership and extrapolate the principles that create cohesive teams and minimize turnover.
-
Electronic Batch Reporting
Learn how a CDMO automatically created a comprehensive batch report model to drive their reporting and speed root cause identification of quality failures with an Industrial DataOps software solution.
-
Key Considerations For Selecting A Solenoid Valve In Health & Science Applications
Solenoid valves are vital in medical devices, enabling precise fluid and gas control. Explore customizable, high-reliability valves that optimize performance while conserving space, weight, and power.
-
Vial Adapter Transfer Device Compatibility With Cell Therapies
Needles and syringes are commonly used to retrieve cell therapies from vials, posing the risk of needle stick injury. Explore the suitability of needle-free polycarbonate transfer devices.
-
How Sharp Packaging's Clinical Packaging Process Is 30% Faster
Discover how digitized workflows and real-time verification are helping streamline clinical trial packaging, reduce complexity, and improve quality in a high-stakes, time-sensitive environment.
-
Addressing The Challenges In Lentivirus Harvest Clarification
Traditional depth filtration struggles with lentiviral harvest clarification, leading to low yields and high costs. However, single-use centrifugation offers a promising alternative.
NEWS
-
Artificial Intelligence Meets Embedded Development With Microchip's MPLAB® AI Coding Assistant2/19/2025
Microchip Technology (Nasdaq: MCHP) is leveraging the power of Artificial Intelligence (AI) to assist software developers and embedded engineers in writing and debugging code with the launch of its MPLAB® AI Coding Assistant.
-
GS1 Brings Trusted Medical Product Information To Smartphones Through Collaboration With Google11/20/2025
GS1, the global organisation behind the barcode, today announced a collaboration with Google that enables GS1 DataMatrix barcodes – already printed on millions of medicine packs worldwide – to be scanned and recognized by Google Lens, the company’s visual search tool.
-
WEROCK Presents Scan Engine And Robust Barcode Scanner With Long Range11/4/2025
WEROCK Technologies GmbH, an innovative manufacturer of robust barcode scanners, mobile data collection devices, and rugged tablet PCs, is expanding its product portfolio with the Rockscan TM2038LR long-range scan engine and the robust Rockscan PX3000 Long Range barcode scanner, which integrates the engine.
-
E-I-E-I-Omics: New Discoveries In Corn Genetics Could Help Grow More Productive, Resilient Crops4/22/2025
By analyzing DNA from different cells in nearly 200 lines of maize plants, research led by the University of Michigan has revealed insights that could help growers better adapt their crops to a fast-changing environment.
-
3D-Printed Helixes Show Promise As THz Optical Materials12/15/2025
Researchers at Lawrence Livermore National Laboratory (LLNL) have optimized and 3D-printed helix structures as optical materials for Terahertz (THz) frequencies, a potential way to address a technology gap for next-generation telecommunications, non-destructive evaluation, chemical/biological sensing and more.