Supporting CRO/Research Services For Your Clinical Trials

From site selection to data management and regulatory guidance, Altasciences' experts orchestrate every aspect of your clinical programs. Expect quality, speed, and seamless collaboration at every step.
Altasciences has been providing clinical research support services to the global biopharmaceutical industry for close to three decades. Our expert teams are fully equipped to support, manage, analyze, and report on studies conducted either here at Altasciences or with external collaborators. Whether as part of a development program or a single study, our research support teams deliver all the complementary clinical research support services needed to complete your projects.
Benefit from Working With a Full-Service CRO
As a full-service CRO, we proactively manage your early-phase to Phase Ib/IIa clinical trials from start to finish. Benefit from a single partner across early phase with project-specific services and focused clinical study management, as well as a single point of contact with the expertise to manage all aspects of your clinical trial.
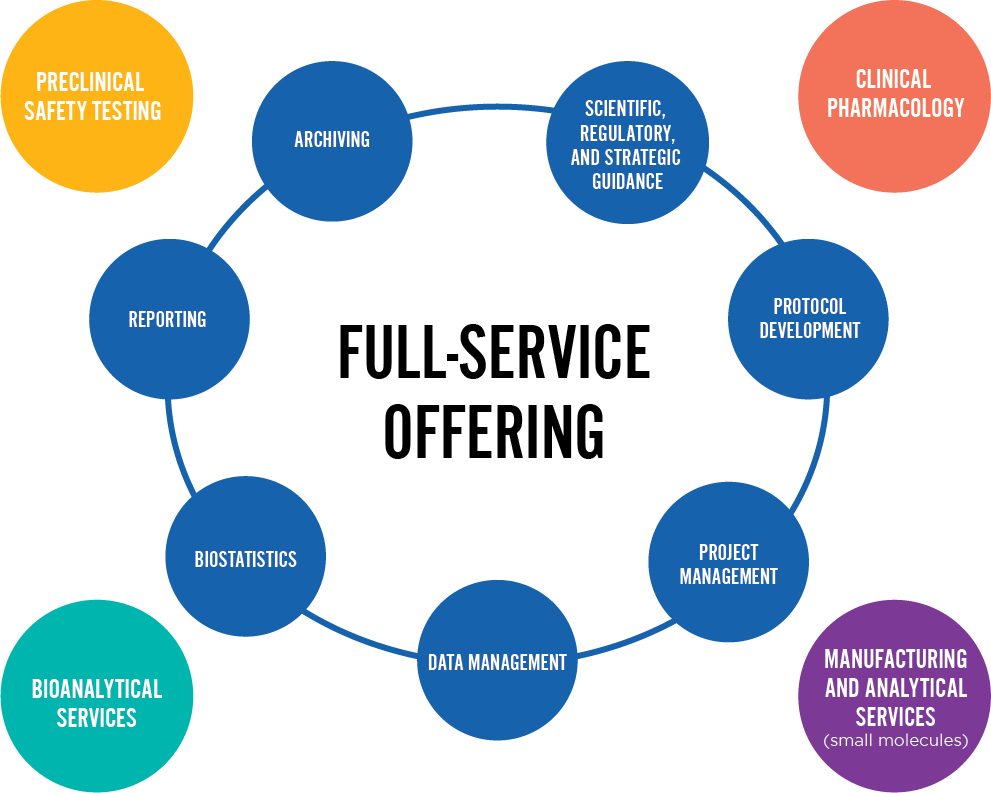
CRO Services
- Clinical Trial Site Identification and Selection
- Clinical Trial Study Start-Up
- Biostatistics
- Data Management
- Scientific Guidance and Regulatory Affairs
- Pharmacokinetics/Pharmacodynamics/Toxicokinetics
- Clinical Trial Site Management
- Clinical Trial Monitoring
- Project Management
- Medical Writing
- Clinical Sample Management Kits
- Full-Time Equivalent (FTE)
Integrated Services
- Laboratory Sciences
- Clinical Research Services
- Manufacturing and Analytical Services
- Nonclinical Services
- Program Management
- Quality Assurance
Clinical Research Support Services
The evolving landscape of drug development continues to reshape our world. Simultaneously, clinical research has become notably more challenging with its share of scientific, operational, and regulatory complexities. Our mission is to support you in safely expediting the development of your therapeutics. We are experts at orchestrating a spectrum of services for optimal accountability, efficiency, and integration. With Altasciences, you can expect quality outputs and efficient operations, as well as less duplication of management oversight on your end.
Our approach synchronizes operational proficiency, medical insights, regulatory knowledge, and the resources of our nonclinical sites, wholly owned laboratories, Phase I units, and manufacturing site. This integrated solution is designed to streamline and enhance your journey, providing an integrated early-phase pathway for accelerated drug development.
