Preclinical Safety Pharmacology Studies
Safety pharmacology is the cornerstone of responsible drug development. It involves the evaluation of a drug candidate's potential impact on vital organ systems such as the cardiovascular, respiratory, and central nervous systems. By identifying any potential safety concerns early in the development process, safety pharmacology studies play a crucial role in minimizing risks to patients and ensuring the overall safety and efficacy of the final product.
Our team of experts conduct safety pharmacology studies in compliance with international regulatory guidelines, including those outlined by the International Council for Harmonization (ICH), S7A and S7B. We ensure that your data meets all regulatory standards, facilitating a smooth transition from preclinical to first-in-human trials.
Safety Pharmacology Core Battery And Supplemental Studies
Our comprehensive safety pharmacology services are designed to assist you in navigating the complex landscape of drug development. Extensive expertise in standalone and integrated repeat-dose designs is indispensable when designing and conducting safety pharmacology and toxicology studies.
Our safety pharmacology services Include:
In Vitro Pharmacology Studies
Utilizing state-of-the-art technology, we conduct in vitro assays to assess the pharmacological effects of your drug on cellular systems including Complete cardiac ion channel assessments such as Na+, Ca++, K+ or hERG, helping to identify potential risks early in the development process.
In Vivo Pharmacology Studies
Our in vivo studies involve the evaluation of your drug in animal models, allowing us to assess its impact on vital organ systems such as the cardiovascular, respiratory, and central nervous systems. Our team of safety pharmacologists is well-versed in the latest technologies and evaluations, including the utilization of implanted and jacketed external telemetry (JET) for continuous collection of electrocardiographic and hemodynamic data in large animals.
With our extensive experience, we integrate safety pharmacology endpoints into general toxicity studies to deliver efficiencies in time and resources, as well as provide certain analytical advantages. In some cases, combination studies can also be a practical approach for safety pharmacology studies.
Altasciences' scientific teams will align with all necessary criteria in designing your integrated studies. We'll ensure that safety pharmacology requirements do not confound interpretation of general toxicology endpoints, and vice versa.
GLP Studies
We offer Good Laboratory Practice (GLP) compliant studies, ensuring the reliability and integrity of your data for regulatory submissions.
Customized Solutions
We understand that every drug development program is unique. Our safety assessment programs are adaptable to suit your project's precise requirements, ranging from non-GLP safety screening assays to GLP safety pharmacology study designs. Whether your project involves small- or large-molecule compounds, we can customize our services accordingly to meet your study goals.
Safety pharmacology evaluations have various applications in early phase drug development. They help to:
- assist in the selection of candidate compounds in early drug discovery;
- evaluate potential risks and adverse effects of drugs in preclinical studies;
- identify signals in preclinical studies that can serve as biomarker candidates in the clinic; and
- explain predicted and unpredicted side effects during clinical development.
Maximize Drug Safety: Our Safety Pharmacology Study Capabilities
A core battery of safety pharmacology tests is required by international regulatory guidelines (ICH S7A/S7B) prior to initiation of the first human dose of an investigational medicine. In some instances, based on scientific rationale, this series of testing should be supplemented.
We offer an integrated approach to safety pharmacology, combining in vitro and in vivo studies to assess the impact of your drug across various organ systems. This comprehensive approach allows for a thorough evaluation of potential risks and enables informed decision-making in preclinical development.
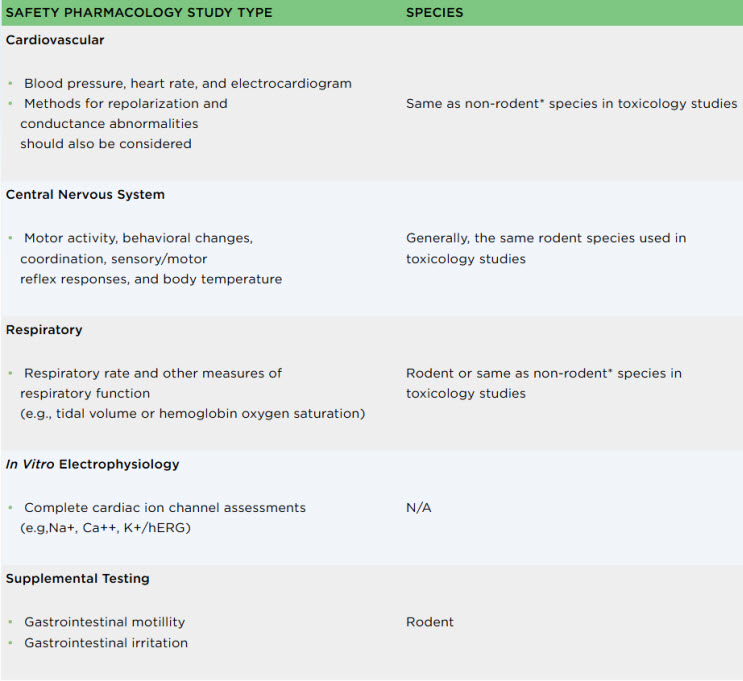
*Respiratory assessments can be combined with cardiovascular evaluations in the non-rodent species using surgically implanted telemetry.
Combination of the cardiovascular and respiratory safety pharmacology assessments in the selected non-rodent species is also a suitable approach, using surgically implanted animals. This combination permits study of time-dependent effects, and insight into possible mechanisms of action. Since two of the three core safety pharmacology assessments are combined, this approach can decrease the number of animals used (3Rs), and reduce cost and other resources.
