Incorporating Antigen Controls Into Your HCP ELISA Workflow
Including Cygnus antigen controls in your HCP ELISA design will generate more robust data, and provide more information regarding assay performance, including the ability to detect variability between runs, and the necessary data integrity for confirming assay results as trustworthy. Here, we’ll describe how antigen controls can help you diagnose any issues should they arise.
Track HCP ELISA performance with Cygnus antigen controls
When performing Cygnus ELISA testing, each assay should include multiple types of controls in order to provide the strongest chance of assay success as well as aid in any potential root cause investigation should you obtain unexpected or inconclusive results. In addition to including negative and positive controls for your sample testing, incorporating Cygnus Antigen Controls is an easy way to help you understand how your data sets compare to one another. Available in high and low concentrations for our most popular kits, these controls serve to establish a benchmark upon which to monitor multiple assay trends over time, including standard curve performance, analyst performance, as well as equipment performance. Keep in mind that regulatory guidelines suggest incorporating at least 3 (ICH, FDA) or 4 (EMA) different known concentrations of HCP antigen into precision and accuracy measurements, so qualifying the assay with antigen controls as part of your workflow will fast-track your assay qualification and validation.
Troubleshooting HCP ELISA performance with Cygnus antigen controls
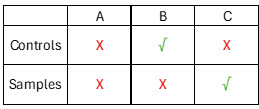
Monitoring the consistency of your antigen control and sample readouts can help diagnose issues with your HCP ELISA. If problems arise with your standard curve, this will impact both the sample and control results of your HCP ELISA. In this case, a significant shift in returned sample results as well as the returned control results will confirm an issue with the standard curve quantitation (scenario A below). On the other hand, if you observe a significant shift in the sample results but not the antigen control result, this indicates that there is an issue only with the samples themselves, and not with the quantitation of the standard curve (scenario B below). However, if you are starting to work with a new kit lot, a significant change in sample results observed without a corresponding change in control results could mean that there is a performance difference in this particular ELISA kit lot versus the HCPs found in your sample. In some isolated cases and depending on the nature of the DS, antibody lot changes could lead to differences in sample results that would not impact the control value, as the antigen mixture in the Cygnus controls is the same as the ELISA kit standards. Again, this case would apply only in rare situations when to starting use of a new kit lot; Cygnus can help troubleshoot this type of data if it arises.
The last case would be when the Cygnus antigen control results fail specifications and the sample results pass (scenario C below); in most cases, this suggests an analyst performance issue or an equipment issue. Situations such as assay drift due to loading time can impact plate readings, and if the controls are loaded further away from the standard curve, this case is more likely due to procedural issues (for more detailed information about how to avoid procedural issues, be sure to check out our previous post on plate loading and washing).