GxP Compliance Software For Life Sciences (21 CFR Part 11)
Accelerated GxP and 21 CFR Part 11 compliance without disrupting your existing workflows.
Regulatory Compliance Solutions for GxP and FDA 21 CFR Part 11
Developing a quality and compliance posture that meets the competing needs of business and auditors is a tall order. Egnyte gives you visibility and control over your most valuable asset—your data, all while adding a layer of regulatory compliance that doesn’t distract from the way you work.
21 CFR Part 11 compliant
When creating, storing, and managing GxP-regulated documents, Egnyte for Life Sciences complies with regulatory requirements, like Part 11/Annex 11. The platform supports audit trails, checksums for data integrity, and robust access control for a simple yet effective path to GxP and 21 CFR Part 11 compliance.
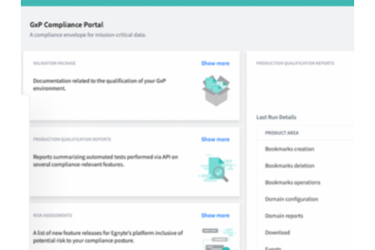
Whether your compliance envelope covers all your documents or just a single type, we make it easy to set up a regulated environment accessible only to credentialed employees and external partners. Egnyte’s GxP compliance portal is a central hub to facilitate auditing, validation, and reporting.
We have streamlined the process for anyone in your organization to initiate, review, and approve controlled documents with Part 11 compliant e-signatures. Flexible enough to handle single- and multi-step workflows, designated stakeholders can collaborate on and approve regulated documents.
