Enrollment Surpasses Projections In International Psoriasis Studies

SPONSOR
A leading global pharmaceutical company selected IQVIA™ Biotech to support four large, international Phase III biologic studies in moderate to severe psoriasis. IQVIA Biotech’s dermatology team was selected because of its proven patient recruitment process with a record of enrollment that could save the sponsor time and money.
CHALLENGE
IQVIA Biotech’s objective was 27% of enrollment per recruitment management participating site while each site was expected to achieve the majority 73% of enrollment. Outside of the United States (U.S.) and Canada (CA), advertising as a means of recruitment for clinical trials is not widely accepted. Also, as most European Union (EU) and rest-of-world (ROW) countries have socialized healthcare, people are less likely to participate in clinical trials. IQVIA Biotech was asked to provide advertising support in countries that may never have used mass media to support patient enrollment in clinical trials. Obstacles that IQVIA Biotech had to overcome included reluctant regulatory authorities, skeptical investigators who initially saw recruitment advertising in opposition to their professional ethics, and a public in each country that had little or no knowledge or experience with recruitment advertising for clinical trials.
SOLUTION
IQVIA Biotech worked closely with investigators and the sponsor to create a Patient Recruitment Management Plan (RMP) that would be accepted by both the regulatory authorities and the general public while not offending competing physicians within the investigational sites’ area. IQVIA Biotech’s patient recruitment process includes predictive enrollment, which offers valuable insights into managing, tracking and reviewing enrollment metrics. Through regular analysis, IQVIA Biotech was able to efficiently adjust recruitment advertising plans as needed for each site. The results exceeded sponsor expectations.
RESULTS
IQVIA Biotech’s efficient management helped ensure the most effective use of the advertising budget, including daily monitoring of the sites’ advertising responses and screening results. Full randomization was completed within the sponsor’s timelines with overall sites’ enrollment exceeding the projected level of 27% for recruitment management participating sites. IQVIA Biotech’s combined four-study average recruitment contribution was 35%, with a high of 44%, and three out of four studies surpassing the projected 27% enrollment goal. In a country-by-country evaluation, IQVIA Biotech surpassed the projections in ten out of 13 countries.
IQVIA BIOTECH’S RMP SURPASSES ENROLLMENT PROJECTIONS
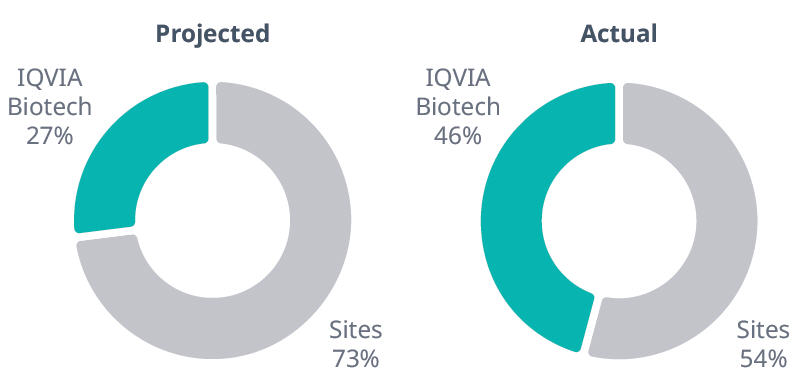
In the EU/ROW portion, IQVIA Biotech’s contribution was 46% of enrolled patients at sites participating in the RMP.
Percent of EU patients enrolled: Actual vs projected
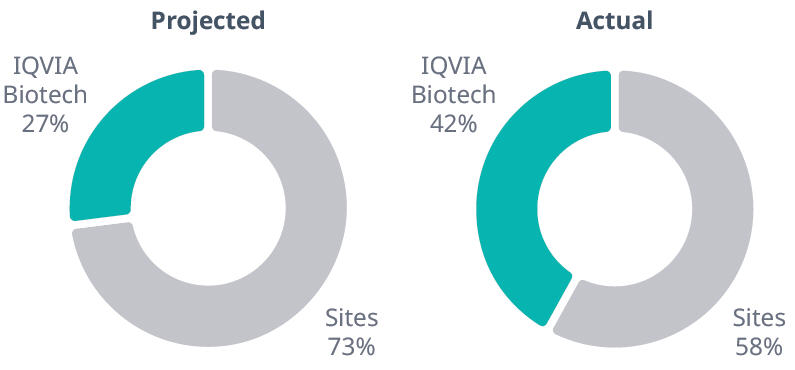
In the U.S. portion, IQVIA Biotech exceeded expectations with an average 42% of enrolled patients at sites participating in the RMP.
Percent of U.S. patients enrolled: Actual vs projected
CONCLUSION
IQVIA Biotech’s patient recruitment process and predictive enrollment expertise can dramatically improve enrollment.

