Effect Of Membrane Filter Pore Size On Microbial Recovery And Colony Morphology

Summary
Membrane filters with a 0.45 µm pore size have long been recognized as the standard for growth of microorganisms. However, there is little published literature comparing the effects of different pore sizes on colony size and recovery.
The 0.45 µm pore size is used to recover bacteria and other microorganisms from many samples and environments—almost to the exclusion of other pore sizes. Only rarely are other sizes used for growth and recovery and there is little information available on the effects of different pore sizes on microorganisms. However, other pore sizes are commercially available for microbial enumeration and users will occasionally substitute a filter with a pore size larger or smaller than 0.45 µm in an attempt to improve their results.
This study takes a broad look at the influence of different pore sizes on some common microorganisms. It provides data on the effect of pore size on growth and recovery. The study compares a variety of microorganism/media combinations on a range of pore sizes: 0.22, 0.45, 0.7, 0.8, and 1.2 µm. The filtration method used in the study was a standard glass funnel and base with vacuum. Test filters were plated on solid (agar) media and compared against spread plates.
There was no universal pattern of results. Some microorganisms, such as Micrococcus luteus and Candida albicans showed no significant difference in recovery or colony size with membrane pore size. Other organisms such as Pantoea agglomerans showed no difference in colony size but had low recoveries on 1.2 and 0.22 µm membrane filters.
Acceptable recovery for membrane filters was defined as being ≥90% versus the controls (spread plates*). The 0.45 µm membrane filters met this definition with all test systems. Some test systems showed equivalent recoveries with other pore sizes but in no case were the results significantly better. The lowest recoveries were seen with extremes of the pore size range (1.2 and 0.22 µm).
Materials and Methods
The pore sizes used in this study were selected from the various sizes of mixed esters of cellulose membrane filters manufactured by Millipore® (Table 1).
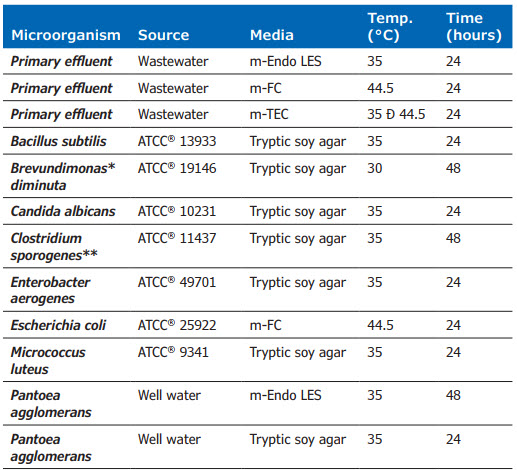
The microorganisms and media combinations used in this study were chosen as a broad representation of common membrane filter applications: pharmaceutical, food and beverage, USP testing, water testing, and general microbiology (Table 2).
As an adjunct to the recovery and colony size experiments, the test filters were tested for their retentive capabilities under the conditions of average use. Each filter was challenged with a low level of Brevundimonas diminuta (the standard organism for retention testing) and the filtrate was retained for enumeration.
Table 1: Test Filters
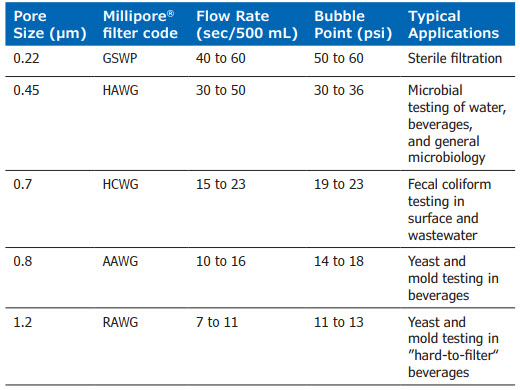
Table 2: Test Method
*Previously grouped in the genera Pseudomonas
**Grown anaerobically using a Gas Pak jar (BBL)
Results
The selection of test methods was not intended to be exhaustive but to give a broad overview of microbial recovery in relation to filter pore size. Six different membrane filter pore sizes were tested with 12 microorganism/media combinations that are representative of the types of microorganisms encountered by those using the membrane filter technique.
Colony Size
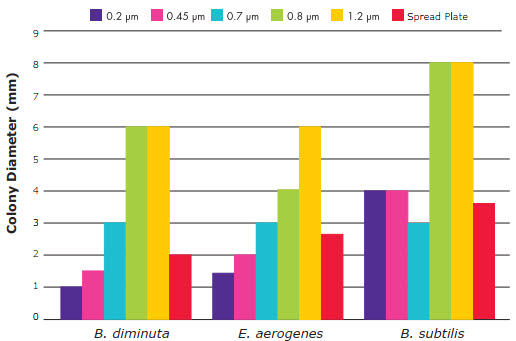
- Three test methods, B. diminuta, E. aerogenes, and B. subtilis, showed differences in colony size with pore size.
- Colonies grown on 1.2 and 0.8 µm membrane filters were larger than colonies grown on other filter pore sizes or spread plates.
- Colonies grown on 0.7 µm membrane filters were the same size as, or slightly larger than, colonies grown on spread plates.
- Colonies on 0.45 and 0.2 µm membrane filters were the same size as, or somewhat smaller than, colonies grown on spread plates.
- Other test methods showed virtually no difference in colony size with any of the other pore sizes as compared to colonies grown on spread plates.
Colony Size Versus Pore Size
Microorganism
Microbial Recovery
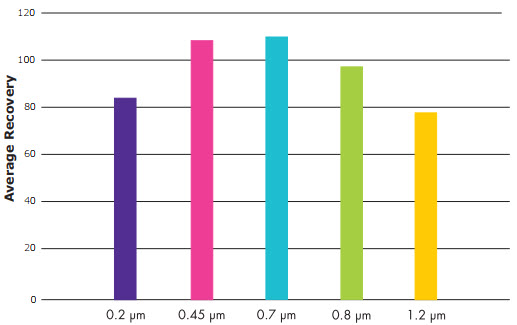
- 0.45 µm and 0.7 µm membrane filters demonstrated equivalent recoveries (≥ 90% versus spread plates) for all 12 test methods.
- Although the average recovery for 0.8 µm membrane filters was acceptable over the 12 experiments, the pore size had lower recoveries than 0.45 and 0.7 µm membrane filters.
- Although 0.22 and 1.2 µm membrane filters gave acceptable recoveries with some systems, their average recovery was significantly lower overall.
Average Percent Recovery for 12 Runs
Pore Size
The average performance of each pore size was determined using all the test systems.
*ISO 7704 requirements state that membrane filters with colony forming unit (CFU) counts ≥80% of the control plate counts are considered acceptable.
Retention
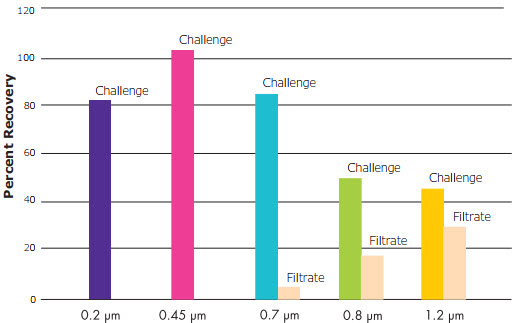
- The larger pore sizes (1.2 and 0.8 µm) allowed significant passage of a small organism at low challenge levels (starved B. diminuta) but there was no passage with the 0.45 or 0.22 µm pore sizes.
- Although 0.22 µm filters retained the challenge, the average recovery across all experiments was lower than 0.45 µm filters.
- Passage might be one reason why larger pore sizes (> 0.7 µm) showed lower recoveries than smaller pore sizes.
Recovery of Challenge Versus Pore Size
Pore Size
Overall
Recovery is much more complex than the retention of microorganisms on the surface of a membrane filter and the influence of pore size. It is a combination of factors that may include:
- The microorganism species and its condition each microorganism has the potential to react differently
- The sieving effects of the pore size as it relates to the retention of specific microorganisms
- Type of medium and selectivity
- Structure and chemistry of the membrane filter
- Environmental conditions (e.g., moisture, incubation, temperature)
The effect of filter pore size on any specific microorganism/ medium combination is not always predictable. If pore sizes other than those indicated by industry standards are used, they should be validated on relevant samples and media and compared to 0.45 µm.
This study confirmed that the standard 0.45 µm pore size is the most appropriate for general microbiological purposes. The 0.45 µm filters gave the most consistent recoveries across a variety of experiments and did not allow passage of the standard 0.2 µm sterilizing filter challenge microorganism, B. diminuta, under typical filtration conditions.
Conclusion
A membrane pore size larger than 0.45 µm can increase flow rate, throughput, and, occasionally, colony size (which makes the colonies easier to count). However, these larger pore sizes may not have sufficient retention for some microorganisms. Therefore, they are not well suited for total count applications.
Larger pore sizes can be used for enumerating specific organisms, such as fecal coliforms or yeast. They can also be used for difficult-to-filter samples where improved throughput or larger sample volumes are needed. In both cases, the filter's retention performance should be documented for the target microorganism(s).
Pore sizes smaller than 0.45 µm have the disadvantage of decreased flow rate, throughput, and, potentially, recovery. Therefore, the greater retentive properties of the 0.2 µm pore size have little benefit for the enumeration of bacteria, yeast, and molds in the variety of liquids considered in this study.
References
- ASTM F838, Standard Test Method for Determining Bacterial Retention of Membrane Filters Utilized for Liquid Filtration.
- PDA Journal of Pharmaceutical Science & Technology. Evaluation of Recovery Filters for Use in Bacterial Retention Testing of Sterilizing-Grade Filters. Vol. 50, N° 3 - J. Carter.
- Pharmacopoeia, USP <71>, EP 2.6.1, ChP 1101, JP 4.06 Sterility Tests.
- Pharmacopoeia, USP <61>, EP 2.6.12, ChP 1105, JP 4.05 Microbial Examination of Nonsterile Products: Microbial Enumeration Tests.
- PDA Journal of Pharmaceutical Science and Technology: Technical Report No. 26, Sterilizing Filtration of Liquids.
- MilliporeSigma study report No. 00016731-PQPSR1.
