Production Scanners
PRODUCTS
-
We believe that QMS software should adapt to you — not the other way around. ETQ Reliance® is more than just QMS software — it’s a comprehensive quality management software system designed to streamline and elevate every aspect of your organization’s quality processes.
-
PHCbi brand’s 14.3 cu.ft. (406 L) microbiological heated and cooled incubator is designed to deliver precise, reproducible environments across a wide temperature range from -10°C to +60°C. Ideal for applications such as microbiology, food and cosmetic stability testing, plant cell culturing, and environmental studies, it supports programmable temperature and lighting protocols. The microprocessor PID controller helps ensures uniformity and stability, while the intuitive LCD interface allows for 12-step, 10-program memory with built-in alarms and backup. A forced air circulation system and energy-saving operation enhance performance and usability.
-
The Natoli NCF-45 Capsule Filling Machine offers unmatched speed, precision, and versatility for efficient powder and pellet encapsulation, maximizing your production output and minimizing waste.
-
Transform your optical systems with Semrock filters - the brightest, most durable, and spectrally sophisticated filters in the market.
-
PHCbi brand's 30.1 cu ft (851L) CytoGrow Reach-in CO2 incubator helps deliver optimal CO2 control and fast recovery without overshoot following door openings thanks to the IR sensor and PID microprocessor controller. Passive decontamination methods such as the patented inCu-saFe® interior and shelving and the optional SafeCell UV light allows cell production to helps ensure a highly accurate, reproducible environment with larger cell culture products and lab equipment while mitigating contaminants.
WHITE PAPERS AND CASE STUDIES
-
Pioneering Cancer Research Meets Unparalleled Veeva Vault Integration
A biotech company revolutionizing cancer treatment faced delays due to a complex, error-prone document review process. Discover how integrating an innovative strategy transformed their workflow.
-
Challenges And Solutions In Lyophilization Development For ADCs
Successful ADC lyophilization requires a robust, individualized process driven by thermal characterization using DSC and FDM to define the critical parameters for stability and efficient manufacturing.
-
SMB Technology: Optimizing API Purification
Explore how Simulated Moving Bed (SMB) technology can enhance API production while overcoming common misconceptions about chromatographic purification.
-
Optimizing DSP Development: Faster Timelines, Lower Costs, And High-Quality Processes
The biopharmaceutical industry faces growing downstream processing challenges in purifying complex biologics efficiently and at scale.
-
Mobile App And Wearable Integration Collects Longitudinal Data
Get an overview of SISCAPA's utilization of eTechnologies, specifically ePRO, and how their partnership with CDS accurately demonstrates the improvement of data collection and analysis in the biotechnology industry.
-
Generate High Quality Data And Execute Full Factorial Design Of Experiments
Learn how a bioprocessing company supplemented their in-house bioreactor capacity and performed large-scale DOE studies using a cloud bioreactor facility.
-
Medical Device Manufacturer Implements A Next-Gen MES
A medical device manufacturer raced to launch a new product in a greenfield facility to overcome tight timelines and compliance challenges by replacing paper SOPs with a dynamic digital solution.
-
Achieving Licensing For A Healthcare Solution
Facing strict regulations and tricky powders, a healthcare client sought an advanced filling solution. Learn how an expert collaboration led to licensed product, boosting precision and productivity.
-
Reducing ADC Timelines With Integrated Development And Manufacturing
ADCs have proven to be highly effective in cancer treatment, but their manufacturing poses challenges. Learn how a CDMO partner can help bring these treatments to patients safely and efficiently.
-
Scaling With The Client In Mind: A Customer-Centric Approach To Project Scale-Up
In today’s fast-moving biopharma landscape, scaling from clinic to commercial takes more than expertise. See how collaboration can transform scale-up and drive confident progress
-
Digitalizing Batch Records In Pharmaceutical Production
Pharma manufacturing embraces cutting-edge automation, yet outdated paper-based documentation hinders efficiency and delays batch releases. See how digital solutions can transform compliance.
-
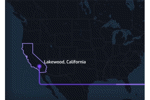
Lakewood Transforms Water Management With Digital Twin Technology
The City of Lakewood, California, embarked on a groundbreaking project to simplify and enhance its water distribution system using Qatium’s innovative digital twin technology.
NEWS
-
Experience Efficient And Flexible Scanning Solutions With Canon's New imageFORMULA R40II Office Document Scanner2/9/2026
Canon U.S.A., Inc., a leader in digital imaging solutions, today announced the launch of the new imageFORMULA R40II Office Document Scanner.
-
Samsung Launches Galaxy Tab Active5 In India: Rugged, 5G-Enabled Tablet Built For Enterprise Workforce8/18/2025
Samsung, India’s largest consumer electronics brand, today announced the launch of the Galaxy Tab Active5 Enterprise Edition in India, a rugged, enterprise-ready tablet engineered to empower businesses and professionals operating in high-intensity environments.
-
MTD Expands Metrology Capabilities To Accelerate Innovation9/29/2025
MTD Micro Molding expands its metrology capabilities with a cutting-edge optical 3D system, enabling faster, traceable, nanometer-precision measurements for complex micro medical components and tooling analysis.
-
Advantest Introduces Advanced Mask CD-SEM 'E3660'9/9/2025
Leading semiconductor test equipment supplier Advantest Corporation (TSE: 6857) today announced the release of its next-generation CD-SEM* E3660, engineered for the dimensional metrology of photomasks and EUV masks used in cutting-edge semiconductor manufacturing.
-
Medication Management Digitalized8/20/2025
The demands made on automated medication management in hospitals are growing continuously.