Business Process Management
PRODUCTS
-
Industry leader in preclinical safety assessments across various therapeutic areas. 30+ years of experience in full range of in vivo non-GLP and GLP studies in both rodent and non-rodent species.
-
Enable secure, flexible data exchange with open REST integrations—streamlining workflows, reducing manual effort, and supporting modern, audit‑ready clinical operations.
-
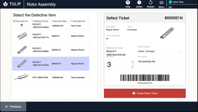
Track and manage defects and non-conformances – such as SOP deviations or missing data – from identification to resolution.
-
Continuous flow processing can be integrated into a broader strategy of sustainable manufacturing. By optimizing processes to reduce waste, energy consumption, and environmental impact, pharmaceutical companies can demonstrate their commitment to environmental stewardship and meet regulatory requirements for sustainable practices.
-
Visualize continuous metabolic changes in cells in real-time and adjust cell culture conditions automatically for enhanced cell quality.
WHITE PAPERS AND CASE STUDIES
-
Converting iPSC To T Cells: Key Challenges And Considerations
Explore how by addressing challenges in differentiation, clone selection, and manufacturing, iPSC-based T cell therapies could revolutionize cancer treatment and other diseases.
-
How A Pharma Company Improved Yield By 1.5% In Just Three Months
A pharma company faced a 4% yield drop and variability at a manufacturing facility. Explore how they leveraged an AI-based platform to unify data, pinpoint inefficiencies, and enhance consistency in yields.
-
CDMO Uses Robotic Gloveless Isolator For Advanced Therapeutics
CDMOs must innovate production architectures to meet the demands of advanced therapeutics to ensure a reliable supply and compliance. Discover how these approaches advance modern medicine and improve patient outcomes.
-
Elevating Downstream Process Development With Real-Time Data Monitoring
Learn how real-time monitoring with PAT enhances process control, shortens development timelines, and supports the shift toward continuous manufacturing in biopharmaceutical production.
-
Mobile App And Wearable Integration Collects Longitudinal Data
Get an overview of SISCAPA's utilization of eTechnologies, specifically ePRO, and how their partnership with CDS accurately demonstrates the improvement of data collection and analysis in the biotechnology industry.
-
Comparative Analysis Of Batch And Continuous Processes
Explore a comparative analysis that showcases the superior performance of continuous processing over traditional batch methods, highlighting key improvements in efficiency and environmental impact.
-
Using Precision Fermentation To Produce Animal-Free Milk Proteins
Learn about an innovative approach to producing animal-free milk proteins using precision fermentation technology supported by bioreactors, offering an ethical alternative to traditional dairy production.
-
Shedding More Light On Ultra-Wideband
Discover how Ultra-Wideband (UWB) technology is revolutionizing manufacturing by providing unparalleled precision in locating products and optimizing processes for enhanced efficiency and safety.
-
How Utilities Can Thrive In The New Generative AI Reality
This white paper explores the transformative potential of generative AI (gen AI) in the water utility sector, demonstrating how it can enhance efficiency, productivity, and decision-making across organizations of all sizes.
-
Enhancing Preclinical Cell Therapy Development
Automation in cell therapy development boosts reproducibility, reduces error, and enhances yield. Learn how it’s reshaping preclinical workflows and accelerating clinical trial readiness.
-
Transformative Innovation: Advancing Drug Development Using In Silico Modeling
Explore how predictive modeling and in silico strategies can turn complex data into actionable insights that accelerate drug development and reduce risk across the development lifecycle.
-
Driving Customer Profitability With Enterprise Platform, Pricing
Explore how the solutions and approaches offered by CDS can help different research stakeholders conduct more cost-efficient clinical trials in the industry's pharmaceutical, biotech, and device sectors.
NEWS
-
25% Of The World's Public-Sector WWTPs To Use AI In 20256/10/2025
As the global population grows and urbanization accelerates, wastewater treatment plants (WWTPs) are facing increasing pressure to deliver high-quality effluent.
-
Emerson Advances Software-Defined Automation With Latest Control System Release1/20/2026
Emerson, a global automation leader, has enhanced its DeltaV Automation Platform through the release of version 16 of its Distributed Control System (DCS) and integrated Safety Instrumented System.
-
Broadcom Launches VeloSky To Deliver Network Convergence, Transform Connectivity3/4/2025
Mobile World Congress 2025—Broadcom Inc. (NASDAQ: AVGO) today introduced VeloSky, a converged networking solution that enables Communications Service Providers (CSPs) to offer integrated fiber, cellular, and satellite connectivity through a single appliance.
-
allGeo, A Service By Abaqus Inc., Enhances Its End-To-End Field Workflow Automation Capabilities3/31/2025
Abaqus Inc., a leader in field service management technology, is proud to announce significant enhancements to its allGeo platform.