Business Process Management
PRODUCTS
-
Planning to launch a patient-centric decentralized clinical trial? Give your clinical trial patients an app that makes it easy to participate—from anywhere at any time.
-
Do you have unknown service lines in your inventory? Avoid sending communications this fall to residents served by unknown lines and learn how 120Water can help you verify.
-
Gene therapies hold immense potential to provide long-lasting treatments for a wide range of diseases. At Cytiva, we offer GMP-compliant, end-to-end manufacturing solutions designed to support the development and commercialization of advanced medicines across multiple modalities – including AAV, adenovirus (AV), lentivirus (LV), plasmid DNA (pDNA) and exosomes. Our dependable suite of technologies and expertise ensures scalability, quality, and regulatory confidence at every stage of your journey.
-
Biotech companies progressing through clinical trials, planning an IPO, and undergoing rapid growth have a unique set of financial, contract, compliance, and reporting needs. We have designed an ERP solution, built on NetSuite, to address all of these needs. With over 50 implementations in the last two years alone, SuiteSuccess for Life Sciences is quickly becoming the defacto software standard for all successful biotechnology companies.
-
Learn how Mycenax is equipped to support process development for a wide range of therapeutic modalities.
WHITE PAPERS AND CASE STUDIES
-
Scaling Success: A Blueprint For Flexible Biometrics Partnerships
Learn how a micro-FSP model and integrated biometrics team can scale from specialized support to a full-service solution, ensuring agility and consistency as clinical trial needs evolve.
-
Comparative Analysis Of Batch And Continuous Processes
Explore a comparative analysis that showcases the superior performance of continuous processing over traditional batch methods, highlighting key improvements in efficiency and environmental impact.
-
Extra-Terrestrial Connections: Enabling Optical Mesh Networks In Orbit
Optical filter implementation into orbital mesh networks must consider how price, performance, and delivery timeline can be optimized together to align with specific customer needs.
-
Pumping And Network Operations Optimized Through Collaborative Digital Twins
Collaborative digital twins streamline utility operations by integrating real-time data into a shared, intuitive environment. This approach enables precise pump optimization, efficient maintenance scheduling, and proactive network simulations to improve water quality and system resilience.
-
FDA Recommendations For Gene Therapy For PMDs
Explore insight into the strategies and FDA recommendations for executing gene therapy development programs, specifically for primary mitochondrial diseases (PMDs).
-
End-To-End Workflow Integration For Antibody Development
Modern antibody discovery generates massive, fragmented datasets that slow collaboration and decision‑making. Examine how unified digital workflows streamline R&D and improve data quality.
-
NuGenesis 9.3 – What's New
Explore the latest features of a user-centric lab management system that comprises data, workflow, and sample management capabilities to support the entire product lifecycle.
-
Enhancing Data Capabilities For Greenville Water Utility
The Greenville Water Utility in Indiana is leveraging Qatium to enhance its water management and leak detection capabilities, significantly improving service efficiency.
-
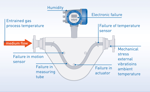
OPTICHECK Technology Built-In – Diagnostics, Verification, And Functional Safety With OPTIMASS Coriolis Mass Flow Meters
State-of-the-art measuring devices such as OPTIMASS Coriolis mass flow meters combine robust measuring principles and powerful electronics to produce a wide range of readings and device-specific data. OPTICHECK technology built-in leverages the features of the devices to translate their available comprehensive measuring system data into sophisticated diagnostics information for plant personnel.
-
Quality Assurance In IVT RNA Vaccine Development Using Electrophoresis
Discover advanced solutions for IVT RNA synthesis, focusing on enhancing fidelity in throughput, innovative technologies, and methodologies to optimize your RNA research and development processes.
-
How Waterloo Harnessed Qatium's Digital Platform
Read about the City of Waterloo, that needed an intuitive, low-cost tool to empower operators without requiring specialized technical expertise.
-
Escondido, CA Uses Cityworks And Esri's ArcGIS® For Its Asset Management System
Read about water utility managers who took advantage of an offer from Trimble to do a pilot program for creating a ‘system of action’ that focuses on leveraging their investment in Esri’s ArcGIS® and water sensor data, location intelligence, and analytics tools.
NEWS
-
Accu-Time Systems And Legion Announce Seamless Time Clock Integration For Workforce Management1/12/2026
Accu-Time Systems, the leader in advanced employee time tracking solutions, is excited to announce that its cloud-based TimeCom Solution now integrates seamlessly with Legion's Workforce Management Platform, bringing new levels of automation, security, and efficiency to enterprise workforce time tracking and scheduling.
-
allGeo, A Service By Abaqus Inc., Enhances Its End-To-End Field Workflow Automation Capabilities3/31/2025
Abaqus Inc., a leader in field service management technology, is proud to announce significant enhancements to its allGeo platform.
-
QNX And Advantech Strengthen Collaboration To Streamline Embedded Systems Development3/20/2025
QNX, a division of BlackBerry Limited and Advantech, a leading provider of embedded platforms and services, today announced an expanded collaboration to better serve their joint-customers across the embedded systems industry.
-
Deloitte Launches AI Solution Suite To Help Organizations Enhance Their Human And Machine Workforce6/24/2025
Deloitte announced the launch of its Human Capital AI solution suite, featuring Workforce Analyzer and Workforce Planner+.
-
IFS To Acquire Softeon, Supercharging The Warehouse Management Systems Category12/17/2025
IFS, the leading provider of Industrial AI software, today announced that it has entered into a definitive agreement to acquire Softeon, a Gartner Visionary and leading provider of cloud-native Warehouse Management, Warehouse Execution and Distributed Order Management solutions.