Healthcare
PRODUCTS
-
As the time, costs and complexity of cleaning validation challenges grow, savvy life sciences companies are building a competitive advantage around smarter approaches to cleaning validation.
-
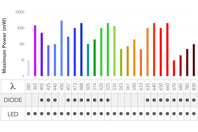
For decades, Melles Griot® Optical Systems have been at the forefront of illumination technology, partnering with researchers and instrument manufacturers to advance the study of biological samples.
-
By removing barriers, and bringing trials to patients where they live, we can expand the geographical reach of clinical research across the globe allowing more patients to participate. Our patient-centric delivery means we can create convenience and comfort working the schedule of the trial around the schedule of the patient and their family, not the other way around. This approach provides important access to medical care, enhances the inclusivity of clinical trials and advances drug development for all.
-
PHCbi brand's 25.7 cu.ft (729L) ultra-low temperature freezer operates on 220V and features VIP Plus vacuum insulated panels to deliver reliable temperature uniformity throughout the chamber. It is ENERGY STAR® Certified and uses only 7.96Wh per day.
-
PHCbi brand's 25.3 cu.ft (715L) ultra-low temperature TwinGuard chest freezer features a dual cooling system to offer our highest level of sample protection through the use of two independent refrigeration systems. The combination of these two circuits work together for fast temperature recovery while keeping ultra-low temperatures if one refrigeration system fails to cool. This freezer is ideal for safe, long term storage of your most high valued products, such as stem cells, embryos, cell lines, rare specimens, and other biological materials.
WHITE PAPERS AND CASE STUDIES
-
Still Using Paper Diaries To Capture PRO Data? Read This.
Access real-world examples of how electronic patient-reported outcome measures (PROMs) optimize data quality and reliability and improve the statistical power of trial data.
-
Designing For Diversity
Embracing diversity enhances our understanding of humanity, fostering empathy and a broader perspective on the world's cultures and behaviors.
-
How To Recruit 236 Patients In An Osteoarthritis Study On Time During The Peak Of The Pandemic
A leading Eastern European contract research organization completed the recruitment of 236 subjects in a Phase 3 osteoarthritis study for Handok Inc. despite hurdles caused by the COVID-19 pandemic.
-
End-To-End Workflow Integration For Antibody Development
Modern antibody discovery generates massive, fragmented datasets that slow collaboration and decision‑making. Examine how unified digital workflows streamline R&D and improve data quality.
-
Enabling Simpler, Quieter, And More Precise Wearables Utilizing Disruptive Pump Technology
Disc pumps enable quieter, smaller, and more precise wearable medical devices, improving patient comfort, measurement accuracy, and system reliability for innovative healthcare solutions.
-
Patient-Focused Data Capture For Cancer Research
In a field where innovation is constant and patient experience is pivotal, oncology demands eCOA solutions designed to balance scientific rigor with human realities.
-
eCOA: Unified Approach Streamlines Processes, Speeds Start Up And Improves Data Quality
Discover how a leading biopharmaceutical company revolutionized its clinical development with an eCOA solution that optimized its processes, cut build times, and enhanced data quality.
-
Working Smarter: Empowering Water Operators With Integrated Data
As water utilities face the challenges of sustainability, efficiency, and service quality, digital technology has become a necessity. The right tech can help deliver reliable service, optimize systems, and meet sustainability goals, but integrating the data streams these solutions generate can offer even greater gains – allowing utilities to move faster and achieve more powerful outcomes.
-
The EMR Interoperability Dream Vs. Clinical Research Reality
Seamless Electronic Medical Record access promises accelerated trials and regulatory-grade evidence, but incomplete, unstructured data requires hybrid strategies using AI and human oversight to bridge gaps.
-
Achieving Self Sufficiency In Data Collection For Optinose
Uncover how this specialty pharmaceutical company was able to gain control over its EDC environment by adopting a solution their team could use in-house.
-
Machine Learning In Intelligent Power Management Systems
Machine learning for MCU implementation (Tiny ML) is transforming battery management and motor control by utilizing machine learning algorithms to analyze sensor data, optimizing performance and assessing system health.
-
Enabling Digital Twins With Computational Fluid Dynamics Modeling
Embrace the transformative power of predictive modeling and digital twin technology to optimize bioprocess efficiency, ensure product quality, and drive innovation in biopharmaceutical manufacturing.
NEWS
-
eClinicalWorks And Sunoh.Ai Assist 75-Provider FQHC Reduce Provider Burnout And Enhance Patient Care10/23/2025
eClinicalWorks, the largest ambulatory cloud EHR, today announced the successful integration of Sunoh.ai, the EHR-agnostic AI medical scribe across Florida-based Suncoast Community Health Centers locations.
-
AMBIR Launches Advanced Healthcare Barcode Scanner Series To Enhance Patient Safety And Clinical Productivity7/2/2025
AMBIR Technology, a leading provider of digital capture technology for businesses and consumers.
-
AmpliTech Group Announces Innovative True 5G Technology Development2/20/2025
AmpliTech Group, Inc. (Nasdaq: AMPG, AMPGW), a designer, developer, and manufacturer of state-of-the-art signal processing components for satellite, Public and Private 5G, and other communications networks, including the design of complete 5G/6G systems and a global distributor of packages and lids for integrated circuits assembly, today announced, it is setting the record straight on what constitutes True 5G and how its revolutionary technology is paving the way for the future of wireless connectivity.
-
Castor Catalyst: Launching Self-Driving Clinical Trials With Google Cloud AI10/20/2025
AI-powered platform built on Google Cloud automates clinical trial tasks, reducing time, cost, and errors while generating regulatory-grade real-world evidence through human-supervised automation.
-
GE HealthCare Collaborates With Two Major Medical Systems To Advance AI Technology Designed To Transform Hospital Operations And Improve Patient Care10/20/2025
GE HealthCare today announced collaborations with two leading U.S. health systems—The Queen’s Health Systems in Honolulu, HI and Duke Health in Durham, NC—to help advance the development of GE HealthCare’s new AI-driven hospital operations software.