Healthcare
PRODUCTS
-
Manufacture high-quality products at any level of compliance.
-
Turning Data into Insights
At Worldwide Flex, we understand that clean data is the foundation of successful analyses. We are an accredited Medidata Partner and certified study builders in multiple platforms.
-
The leader in digitizing validation
Setting the digital validation standard for 20 years, ValGenesis VLMS is used by 30 of the top 50 global life sciences companies.
The gold standard of standardization
Strengthen your compliance stance and lower the cost of quality with enforced standardization and absolute data integrity.
Knowledge integrity to data integrity
Gain total peace of mind with a single source of validation truth with documentation aligned to ALCOA+ standards.
One platform, boundless scale
Start small or start global. VLMS scales effortlessly to support new systems, new sites, new products, new languages, and new validation processes.
-
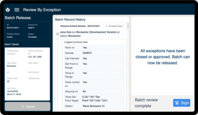
Enforce standardized workflows while collecting data in real time, giving you the visibility you need to prevent deviations, review exceptions, and release batches faster.
-
With a portfolio of over 5,000 recombinant proteins and an industry-leading, scale-up ready protein development platform, ACROBiosystems has accumulated over 10 years of experience in developing recombinant proteins.
WHITE PAPERS AND CASE STUDIES
-
Decentralized And Community-Based Solutions Driving Women's Healthcare
Clinical trials should reflect the affected population by reducing participation barriers and bringing trials to patients to increase inclusion and improve real-world data.
-
Forge Boosts Efficiency And Compliance During External Collaboration
Streamlined collaboration and unified quality systems are helping CDMOs cut review times. Discover how connected workflows improve compliance and efficiency for faster delivery of life-changing treatments.
-
Designing For Diversity
Embracing diversity enhances our understanding of humanity, fostering empathy and a broader perspective on the world's cultures and behaviors.
-
Achieving Self Sufficiency In Data Collection For Optinose
Uncover how this specialty pharmaceutical company was able to gain control over its EDC environment by adopting a solution their team could use in-house.
-
Simplify CAPA In 7 Steps
Discover how to streamline corrective action/preventive action (CAPA) management in regulatory environments in 7 steps.
-
Electronic Batch Reporting
Learn how a CDMO automatically created a comprehensive batch report model to drive their reporting and speed root cause identification of quality failures with an Industrial DataOps software solution.
-
Scaling With The Client In Mind: A Customer-Centric Approach To Project Scale-Up
In today’s fast-moving biopharma landscape, scaling from clinic to commercial takes more than expertise. See how collaboration can transform scale-up and drive confident progress
-
Streamline Your mAb Manufacturing With Strategic Development And CDMO Compatibility
Though the journey to mAb development begins at lab scale, for clinical trials and commercial distribution, a production process must be streamlined and scalable to 2,000 L production and above.
-
How Syneos Health Tackles Protocol Complexities
Explore solutions employed by Syneos Health, a global contract research organization, to optimize Phase IIV clinical trials, particularly under a complex adaptive trial protocol.
-
Best Practices For Sterilization Validation In Medical Device Manufacturing
Sterilization validation ensures medical device safety by confirming sterility through rigorous testing, method selection, and regulatory compliance, using both established and emerging sterilization technologies.
-
Quality: The Link Between Platform, Processes, And Patients
Quality management in healthcare and life sciences is crucial for optimizing patient health. Learn how modern quality management systems optimize patient outcomes, enhance safety, and drive operational efficiency.
-
How Medidata Link Is Supporting Moderna's Clinical Trial Real-World Data Linkage Initiatives
Discover how Medidata Link empowers Moderna's clinical trial RWD linkage initiatives, enhancing insights into treatment outcomes while minimizing administrative burdens.
NEWS
-
Study Finds Coastal Flooding More Frequent Than Previously Thought6/4/2025
Flooding in coastal communities is happening far more often than previously thought, according to a new study from North Carolina State University and the University of North Carolina at Chapel Hill. The study also found major flaws with the widely used approach of using marine water level data to capture instances of flooding.
-
My Visit Revolutionizes Field Management With Accurate Visit Tracker And EVV Tools6/5/2025
In an increasingly mobile and compliance-driven world, My Visit introduces a transformative solution for businesses and service providers that need to track field activity, manage mobile teams, and meet regulatory standards.
-
SMS360 Enhances EHS Management With New Offline Capabilities For Waste, Energy, Warehouse, And Construction Industries2/12/2025
SMS360, a leader in digital safety solutions, proudly announces the launch of enhanced offline capabilities designed to revolutionize safety and compliance management in the waste, energy, warehouse, mining and construction sectors.
-
IFS To Acquire Softeon, Supercharging The Warehouse Management Systems Category12/17/2025
IFS, the leading provider of Industrial AI software, today announced that it has entered into a definitive agreement to acquire Softeon, a Gartner Visionary and leading provider of cloud-native Warehouse Management, Warehouse Execution and Distributed Order Management solutions.
-
AMBIR Launches Zapier Integration For AmbirScan Software, Enabling Seamless Workflow Automation12/5/2025
AMBIR, a leading provider of document scanning solutions, today announced the launch of Zapier integration for its AmbirScan software platform.