Drug Master File

What is included in Forge's DMF?
DMFs come in all shapes and sizes, but Forge's DMF includes the following components:
- Information on our critical raw materials, including our Ignition Cells™ and pEMBR™ adenovirus helper plasmid
- An overview of our platform AAV manufacturing process
- Process controls
- Facility layout and engineering information
- And more!
How does Forge's DMF improve timelines?
Our DMF can tremendously improve timelines and resources for programs that are entering the clinic. Forge ensures that the DMF has helped to de-risk any client's IND application and can save them months around the submission of an IND. In addition, clients can spend less time or resources to rewrite CMC information from a small library of manufacturing documents and records. There's no need to reinvent the wheel. We've already authored and vetted the documentation through the FDA.
What differentiates Forge from other CDMOs?
Forge is unique because we have our pipeline program. As such, we are securing the necessary FDA approvals for our manufacturing process and facility long before our clients need them. Clients can also have confidence that the DMF has been reviewed and the CMC information has been adopted under a prior program – our internal pipeline program.
What are the main components of an IND?
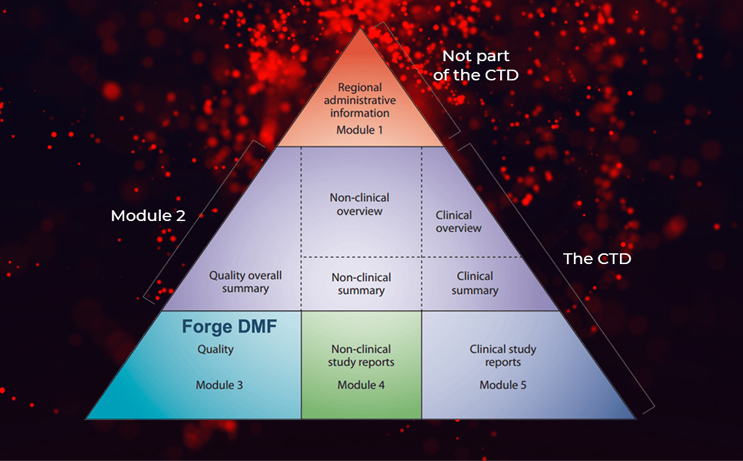
- Module 1: Regional administrative information
- Module 2: Overall summary and overview
- Module 3: Quality information
- Module 4: Non-clinical animal information
- Module 5: Clinical information
Our Forge DMF provides most of the critical components of Module 3, saving time and effort in compiling that section.
How can gene therapy developers set themselves up for better FDA review outcomes?
Here are some tips for setting up for success with the FDA:
- First, have a good communication strategy with DMF holders. Forge is not just a DMF holder – we’re also a CDMO – so good communication is built into our DNA.
- Second, interact early and often with the FDA through pre-IND or INTERACT meetings.
What are other ways Forge provides regulatory support?
- Review and authoring of client CMC documents
- Review and authoring of any regulatory documents
- Assist with FDA requests for information
- Participation in formal meetings
- Ad hoc consulting services
- Electronic Common Technical Document (eCTD) compliant publishing and submission services
