Custom Media And Serum- Support Your Pipeline From Discovery To Commercial Production
Expertise and access to a vast product portfolio to support your pipeline from discovery to commercial production.
Custom Media and Serum from CorningFrom cell culture and antibody production to vaccine development and drug discovery, our high-quality custom media development and manufacturing services can produce customized, tailored media and reagents to meet your unique research needs.
Custom media products are manufactured under the current ISO 13485 standard and FDA Quality System Regulation 21 CRF 820, current good manufacturing practices (cGMP), FDA-registered facility for Class 1 Medical Devices, we offer industry-leading media products and process quality that meets all cGMP and FDA requirements for manufacturing and sterility.
Custom media and serum from Corning, can provide you with a full range of capabilities to meet your custom media formulation, packaging, and regulatory requirements and support you with experience and expertise along the way.
A system that ensures success Our custom media and serum products follow strict production procedures, and are required to meet high- purity and production consistency standards in order to deliver the highest-quality media products. We offer exceptional quality and traceability, including:
- ISO certification: Certified to ISO 13485, which meets requirements related to device design, manufacturing process, supplier management, traceability, and record retention.
- Quality testing: To ensure we meet stringent release criteria, our finished products are tested by industry standards using USP or EP monographs as applicable to ensure they meet functional, biological, and physicochemical requirements
- Sterility Assurance Level: All Corning® media products meet the sterility assurance level of 103, and we constantly seek to provide a sterility assurance that is as low as is reasonably possible
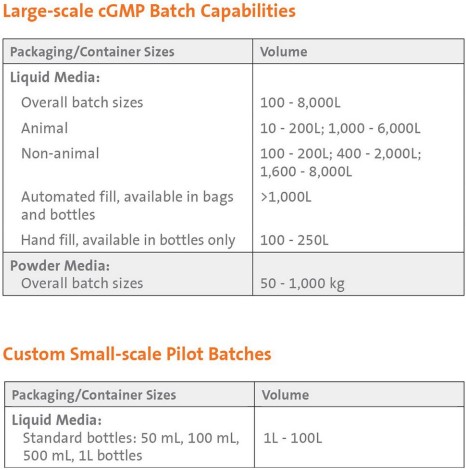
Manufacturing Capabilities
Our manufacturing facilities are committed to delivering high quality, customized products designed to your specifications with:
![]() Rapid set-up times
Rapid set-up times- On-time delivery
- Continuity of supply
- Consistent product performance
- Reproducible processes
Our custom manufacturing capabilities include:
- Separate designated rooms for animal and non-animal products
- Manufacturing within environmentally controlled spaces
- Formulation and dispensing to requested volume specifications, hand-fill, semi-automated, and automated filling
Customizable Components
- USP, EP, and/or JP compendial
- Formulation
- Raw materials
- Unit size and fill
- Powder or liquid
- Animal or non-animal
- Packaging (bags, bottles, shipping packing, kitting)
- Labeling
- Intended use statement within regulatory guidelines

 Rapid set-up times
Rapid set-up times