Sterile Fill/Finish
AbbVie’s new aseptic fill / finish vial line located in Sligo, northwest Ireland, is approximately 130 miles from Dublin. This state-of-the-art line supports lyophilization for potent and biologics along with liquid fill vials. Equipment installation is underway with production commencing H2 2020.
Leverage our experience to give your product an inside edge. Our vast knowledge is based on commercial success that we then apply to find the right solution tailored to meet your product's needs. Our latest offering includes fill/finish capabilities in:
Development (NON-GMP)
Lyophilization process optimization, scale-up and tech transfer
- Computational fluid dynamics (CFD) for modeling to GMP lyophilization for seamless tech transfer
- Differential scanning calorimetry (DSC) and Freeze Dry Microscopy (FDM) for understanding critical product temperatures
- Optimize lyophilization cycle and cake quality
- Minimize development cost via fewer engineering runs at scale
Lab scale equipment
- IMA and SPCScientific
- Manual or peristaltic filling
- 10–500 vials per batch
Clinical And Commercial Manufacturing
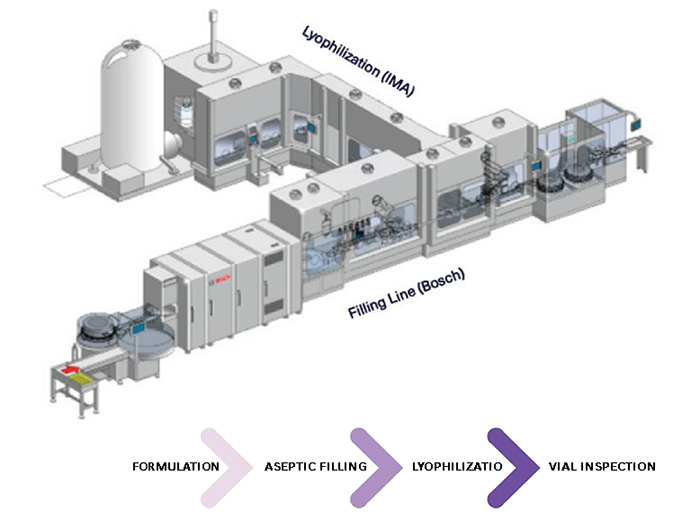
Vial filling
- Bosch aseptic 6 head fill line utilizing peristaltic pump technology
- Single use fluid path for filter
- Line equipped for 6R, 10R and 20R (capable of 2R – 50R)
- Line speed up to 225 vials per minute
- Containment level < 10 ng/m3
- 100% in process weight checks
- Designed for minimal line loss
Lyophilization
- IMA autoload/unload lyophilizer to eliminate human intervention
- 23m2 lyophilization chamber capacity with single batches up to 53,000 vials (6R)
- Space to add 2nd lyophilizer
Finishing/Inspection
- Automated bad crimp detection
- Manual and semi-automatic visual inspection
- 100% oxygen headspace analysis
Analytical
- Development and investigative testing
- HPLC (SEC, IEX, affinity, reverse phase)
- Host-cell protein
- Electrophoresis (CE, IEF, SDS-PAGE)
- ELISA
- Peptide structure determination (LC, MS)
- Oligosaccharide analysis
- NA qualification (threshold)
- DNA identification (PCR)
- Spectrophotometry (FT-IR, UV VIS)
- Bioburden / Endotoxin
- HIC for DAR
- Vial analytical capabilities
- Head space analyzation for H2O and O2
- Container closure development and validation
- Sub-visible particulate
AbbVie
This website uses cookies to ensure you get the best experience on our website. Learn more
