GENiSYS® R Robotic Multi-Format Filling and Closing System
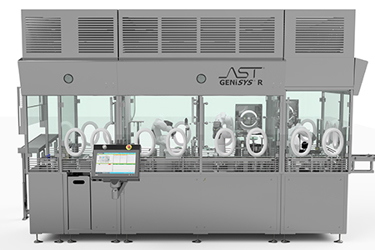
GENiSYS R has a unique blend of automated capabilities to ensure your sterile products, whether clinical or commercial, are processed in strict accordance with cGMP requirements. The system has the flexibility to adapt to your process requirements and container options alike. Its ability to process all ready-to-fill vial, syringe, and cartridge formats allows you to bring drug products to market cost-effectively.
GENiSYS R can be configured with advanced system features and capabilities to tailor the equipment for each unique application. The system meets the highest standards for aseptic processing by providing features such as environmental monitoring, multiple product dispense system options, in-process fill weight control, and electronic batch record reporting.
Clinical and Small Commercial Batch Capabilities
The GENiSYS R is easily configured to process all ready-to-use, nested, vial, syringe and cartridge formats for clinical and small-batch commercial applications. The use of highly reliable robotics enables a complete system changeover in as little as thirty minutes. The system can include automated lyo loading and unloading.
- Vial Filling, Closing, and Sealing
- Syringe Filling and Closing
- Cartridge Filling and Closing
- Lyo or Lyophilizer Integration
Flexible & Modular System Design
In order to meet a wide range of customer and application needs, the GENiSYS R has a modular design that provides our customers a system that is tailored to their application requirements without the cost and risk associated with customization. Available modules include:
- Manual (MBO) or Semi-Automatic Bag Opening (SABO)
- Manual Tub Opening (MTO)
- Automatic Tub Opening (ATO)
- Automatic Filling & Closing (FCM)
- Lyo Prep (LPM)
- Vial Sealing (VSM)
Aseptic Barrier Technologies
GENiSYS R is integrated with isolator-barrier technologies to fully enclose and tightly control the aseptic environment for ideal conditions for processing sterile drug products. The system can be configured with a Restricted Access Barrier System (RABS) or an aseptic isolator to provide uninterrupted aseptic conditions during production. With isolator integrated systems, the GENiSYS R is completely compatible with repeated in-situ bio-decontamination using hydrogen peroxide to further enhance the sterility assurance of the system.
- Restricted Access Barrier System (RABS)
- Isolator – with in-situ bio-decontamination using hydrogen peroxide (VHP)
Maximize Product Yield
Eliminating waste is the key to successful small-batch processing. Necessary operations such as pump calibration, in-process fill weight checks and residual product hold-up in the fluid path all contribute to product rejects. The GENiSYS R’s in-process control system allows for 100% fill weight inspection with minimal reduction in processing speeds to minimize or even eliminate improperly filled containers and minimize product hold-up at the end of the batch – resulting in maximize product yield. In addition, the ASTView Electronic Batch Record (EBR) system provides a complete record of the dispensed material.
Advanced System Features:
- In-Process Control (IPC) that provides real-time fill weight feedback and control - maximizing product yield
- ASTView a high-resolution interface that provides intelligent and intuitive control and real-time process monitoring of the system
- Robotic processing provides clean, reliable and flexible filling and closing of Ready-To-Use, nested, pre-sterilized Vials, Syringes, and Cartridges
- Process flexibility that provides multiple dispense and closing options for syringes, vials, and cartridges
- Aseptic Barrier options allow the system to be integrated with either Isolator or RABS technology to ensure an aseptic environment
- Electronic Batch Report (EBR) System records critical process information that can be used to create 21 CFR Part 11 compliant batch reports
Applications:
- Small to medium batch production of commercial and clinical materials
- Biologics, proteins and potent products
- Multi-product aseptic filling facilities
- Personalized medicine
- Pilot-scale cGMP manufacturing
